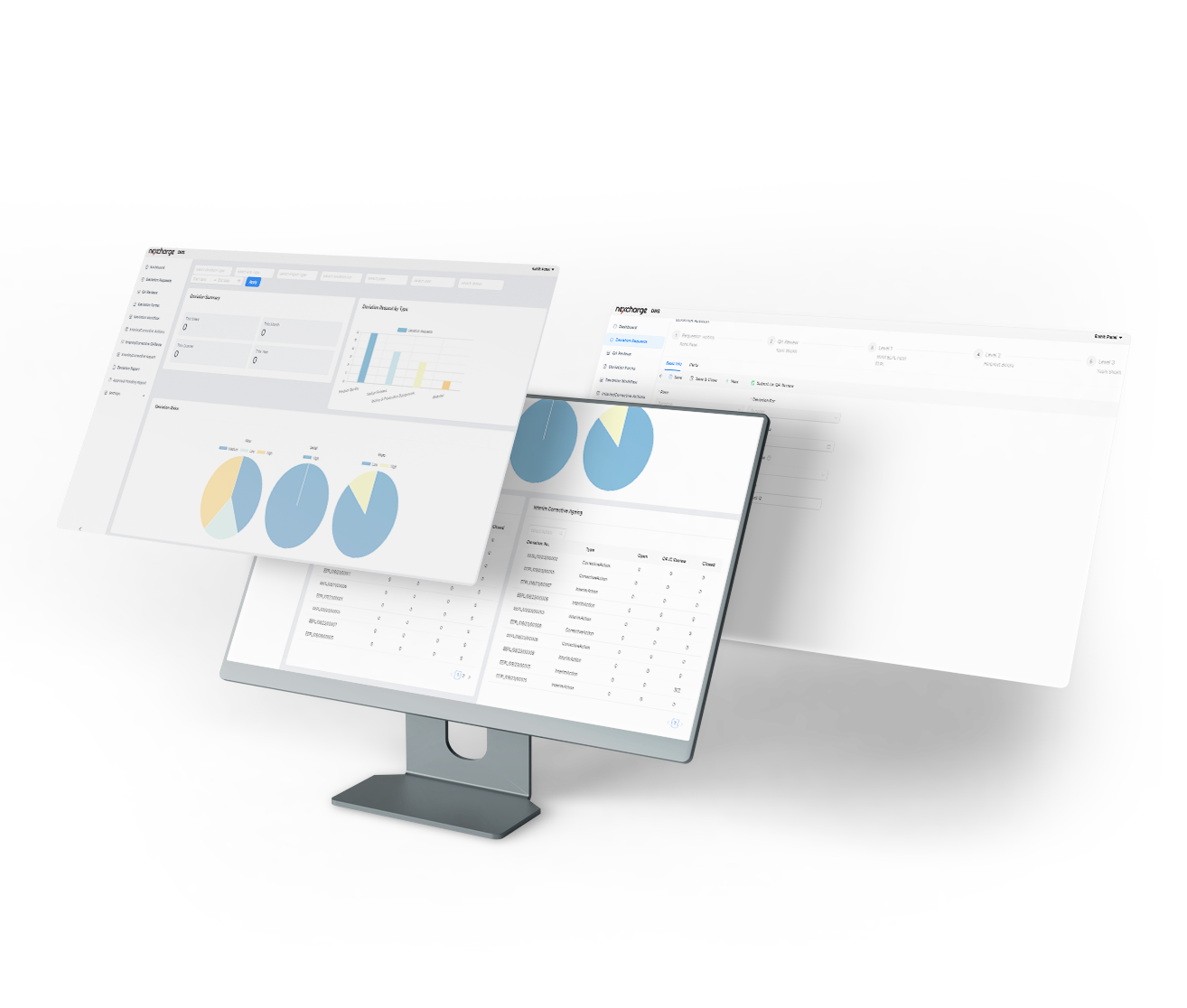
Quality Deviation Management
Background:
Effective deviation handling is a critical aspect of any industry, essential for maintaining the highest standards of quality and compliance with regulations. Our cutting-edge technology is designed to streamline your business processes, reduce deviations, and empower you to take data-driven actions for continuous improvement.
Benefits:
- Improved Quality Control: Ensure product and process quality by identifying and addressing deviations promptly.
- Regulatory Compliance: Stay compliant with industry regulations and standards.
- Risk Mitigation: Proactively manage risks associated with deviations to prevent potential issues.
- Data-Driven Decisions: Make informed decisions based on comprehensive deviation data analysis.
- Process Optimization: Identify areas for process optimization and efciency gains.
- Continuous Improvement: Drive continuous improvement efforts by learning from deviations
Features:
- Web Application: Access the system conveniently through a user-friendly web interface.
- Workow Tracking: Track deviations through the entire process, from identication to resolution.
- Quality Reviews: Conduct quality reviews to assess the impact and severity of deviations.
- Root Cause Analysis: Dive deep into deviations to identify their root causes, facilitating effective corrective actions.
- Interim Actions and Workarounds: Implement interim measures to minimize the impact of deviations while investigations are underway.
- Corrective Actions Against Targets: Dene and track corrective actions to address deviations and meet quality targets.
- Active Email Notications: Stay informed with real-time email notications for important deviation-related events.
- Interactive Dashboards: Gain insights through interactive dashboards that provide a visual representation of deviation data.